Direct synthesis of highly functionalized furans from donor–acceptor cyclopropanes via DBU-mediated ring expansion reactions†
Abstract
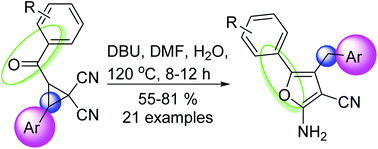
A DBU-mediated, unprecedented formal ring expansion reaction of 2-acyl-3-arylcyclopropane-1,1-dicarbonitriles for the synthesis of multisubstituted furan derivatives is reported. This transformation represents the regioselective ring-opening reaction of cyclopropane-1,1-dicarbonitriles and annulation using an intramolecular addition cascade reaction protocol for the synthesis of fully substituted furans includes use of readily available starting materials, mild reaction conditions, and it is transition-metal catalyst free, has good functional tolerance, and broad substrate scope.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...