Structures and biological activities of cycloheptamycins A and B†
Abstract
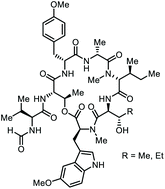
The heptadepsipeptide cycloheptamycin A was isolated from the terrestrial Streptomyces sp. Tü 6314. Its constitution was elucidated on the basis of NMR spectroscopic experiments and mass spectrometric analysis. Its stereostructure was investigated by peptide hydrolysis and derivatization and firmly established by X-ray structure analysis. In addition to the parent compound, a new cycloheptamycin analog, cycloheptamycin B, was discovered and structurally assigned using comparative MS/MS experiments and NMR. The biological profile of both compounds was investigated, revealing a selective inhibitory potential of cycloheptamycins against Propionibacterium acnes.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...