Controlling the regioselectivity of the ring opening of spiro-epoxyoxindoles for efficient synthesis of C(3)–N(1′)-bisindoles and C(3)–N(1′)-diindolylmethane†
Abstract
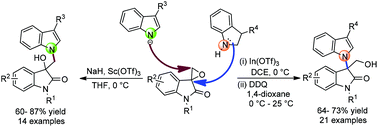
An efficient strategy for the construction of both C(3)–N(1′) bisindoles and C(3)–N(1′) diindolylmethane has been explored via proper tuning of the nucleophilicity of indoline/indole to spiro-epoxyoxindole. Lewis acid-catalyzed highly regio- as well as chemoselective coupling at the C-3 centre of spiro-epoxyoxindoles with indolines furnishes C(3)–N(1′) bisindoles whereas base mediated and Lewis acid-catalyzed regioselective coupling at the less hindered site of spiro-epoyoxindoles with indoles via the SN2 mechanism provides C(3)–N(1′) diindolylmethane.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...