Facile synthesis of chiral ε-sultams via an organocatalytic aza-Friedel–Crafts reaction†
Abstract
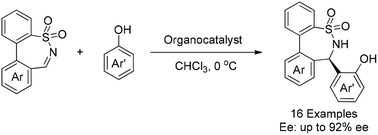
Chiral ε-sultams, with their unique strain cyclic structure, are a type of molecule with important biological activities. A facile enantioselective aza-Friedel–Crafts reaction of seven-membered cyclic N-sulfonylimines with naphthols was developed with a cinchona alkaloid-based bifunctional organocatalyst, giving chiral ε-sultams with an enantiomeric excess of up to 92%.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...