A simple route towards the synthesis of 1,4,5-trisubstituted 1,2,3-triazoles from primary amines and 1,3-dicarbonyl compounds under metal-free conditions†
Abstract
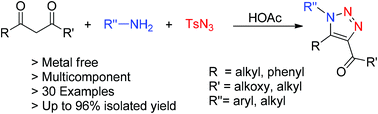
An acetic acid-promoted approach that enables the synthesis of 1,4,5-trisubstituted 1,2,3-triazole derivatives has been achieved. This transformation employs readily available primary amines, 1,3-dicarbonyls and tosyl azide as the starting materials via a cycloaddition reaction under metal-free conditions. The reaction provides a simple access to fully substituted 1,2,3-triazoles from commercial substrates in moderate to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...