meso-Aryl substituted stable unorthodox 5,10-porphodimethenes with α,β and β,β-N-methyl pyrrole connectivities: synthesis and spectroscopic, solid state and theoretical characterization†
Abstract
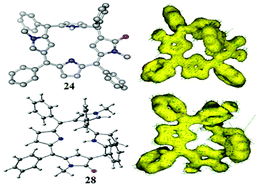
A concise and convenient synthetic methodology leading to an unambiguous isolation of two hitherto unknown highly stable single conformers of meso-aryl substituted unorthodox 5,10-porphodimethenes has been developed by the inclusion of N-methyl pyrrole units with α,β and β,β-linkages into the core of heterocyclic macrocycles.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...