Palladium-catalyzed carbonylative synthesis of substituted cyclopentenones from aryl iodides and internal alkynes†
Abstract
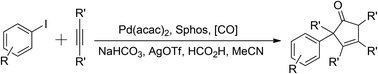
In this Communication, a palladium-catalyzed carbonylative synthesis of substituted cyclopentenones has been developed. With aryl iodides and internal alkynes as the substrates, via a domino process consisting of a formal Pauson–Khand reaction, good yields of the desired products were obtained. Interestingly, formic acid has been used both as a hydrogen source and a carbon monoxide source in this system.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...