Metal-free synthesis of gem-silylboronate esters and their Pd(0)-catalyzed cross-coupling with aryliodides†
Abstract
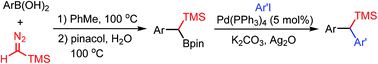
A transition-metal-free method for the synthesis of gem-silylboronate esters with arylboronic acids and trimethylsilyldiazomethane (TMSCHN2) has been developed. This transformation is a straightforward homologation of arylboronic acids and features wide substrate scope and good functional-group tolerance. The gem-silylboronate esters undergo efficient Suzuki–Miyaura cross-coupling with aryliodides and the silyl group of the product can be further functionalized. Tertiary carbon centers with different substituents can be constructed successfully by selective and sequential functionalization.
- This article is part of the themed collections: Synthetic methodology in OBC and Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...