Mono-selective β-C–H arylation of N-methylated amino acids and peptides promoted by the 2-(methylthio)aniline directing group†
Abstract
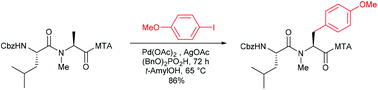
2-(Methylthio)aniline (MTA) directed C(sp3)–H functionalisations are efficient and straightforward protocols for the selective β-modification of N-methylated amino acids. The decreased reactivity of MTA in comparison with the 8-aminoquinoline (AQ) directing group allows for selective monoarylations in high yields without the formation of side products. The protocol is also suitable for the introduction of highly functionalised side chains onto the C-terminal alanines of dipeptides. The MTA directing group can easily be removed, providing free carboxylic acids as valuable building blocks.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...