Synthesis of 2-alkyl-2-boryl-substituted-tetrahydrofurans via copper(i)-catalysed borylative cyclization of aliphatic ketones†
Abstract
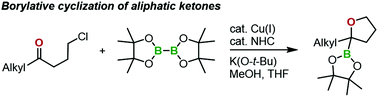
A new method was developed for synthesizing 2-alkyl-2-boryl-tetrahydrofuran derivatives from aliphatic ketones using a copper(I)/N-heterocyclic carbene complex catalyst. This reaction presumably proceeds through the nucleophilic addition of a borylcopper(I) intermediate to ketone, followed by intramolecular substitution of the resulting alkoxide for the halide leaving group. The new borylation products, 2-alkyl-2-boryl-tetrahydrofuran derivatives with a condensed structure around the C–B bond, cannot be synthesized by other methods.
- This article is part of the themed collection: Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...