BODIPY analogues: synthesis and photophysical studies of difluoro boron complexes from 2-aminotropone scaffolds through N,O-chelation†
Abstract
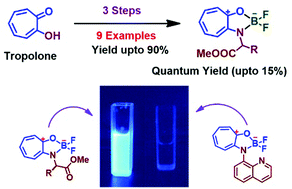
Boron-dipyrromethene (BODIPY) fluorophore derivatives were prepared from a Pyrromethene scaffold and di-fluoroboron. To add to the range of biocompatible fluorophores, this report describes the synthesis and photophysical studies of carboxylate-functionalized BODIPY analogues from natural products, Tropolone and α-amino acids, through N,O-chelation. The structure of a few derivatives was confirmed by single crystal X-ray studies. UV/fluorescence studies revealed their fluorescence behaviors in organic solvents with a quantum yield of ∼0–15%, which varied based on the structure of amino acids. Further, electrochemical studies were accomplished by cyclic voltammetry, which confirm only one irreversible reduction reaction with Epc = −1.3 (V). Finally, their HOMO and LUMO molecular orbitals/energy differences were calculated from a theoretically (DFT) optimized structure, which also supported the role of amino group residues in the enhancement of the fluorescence. The glycinate derivative exhibited the greatest quantum yield compared to the other amino groups. Hence, 2-aminotropone containing BODIPY analogues are potential candidates for the development of various types of functionalized fluorophores as BODIPY analogues.


 Please wait while we load your content...
Please wait while we load your content...