Synthesis and evaluation of photo-activatable β-diarylsydnone-l-alanines for fluorogenic photo-click cyclization of peptides†
Abstract
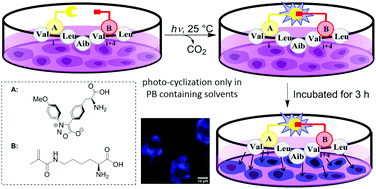
Herein, we design and synthesize a series of photoactivatable β-diarylsydnone-L-alanines (DASAs), which have excellent photo-reactivity with high fluorescence turn-on toward alkenes in a biocompatible environment. The environmental sensing properties of the resulting fluorescent pyrazoline-alanine facilitate its probing capability. By introducing the DASA residue on the side chain of linear peptides, the macrocyclic peptides resulting from the in situ photo-cyclization toward the alkene residue exhibited fluorogenic translocation through live cell membranes.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...