A computational study on H2S release and amide formation from thionoesters and cysteine†
Abstract
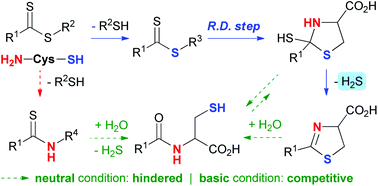
The recognition of the biological activity of H2S has drawn much attention to the development of biocompatible H2S release reactions. Thiol-, particularly cysteine-triggered systems which mimic the enzymatic conversion of cysteine or homocysteine to H2S have been intensively reported recently. Herein, a density functional theory (DFT) study was performed to address the reaction mechanism of H2S release and potential amide bond formation from thionoesters and cysteine to gain deeper mechanistic insights. Three possible mechanisms were considered and we found that the one starting from the nucleophilic addition of the ionized mercapto of cysteine on thionoester to generate a dithioester intermediate (Path A) is kinetically favored over the others starting from the nucleophilic addition of the amine of cysteine to generate thionoamide intermediates (Paths B and C). Dithioester then undergoes intramolecular nucleophilic addition of an amine group and the rate-limiting water-assisted proton transfer to generate a cyclic thiol intermediate, and finally affords H2S and dihydrothiazole via water-assisted elimination. The hydrolysis of thionoamide or dihydrothiazole to produce amide is highly difficult under neutral conditions but is operative under strong basic conditions, which explains the experimental observation that dihydrothiazole rather than amide is the major product. Meanwhile, the ring opening reaction of the cyclic thiol intermediate to form the more stable thionoamide is detrimental to H2S release and becomes competitive under basic conditions.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...