A straightforward TBHP-mediated synthesis of 2-amidobenzoic acids from 2-arylindoles and their antimicrobial activity†
Abstract
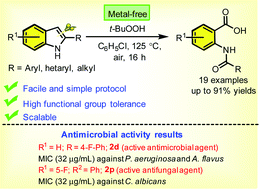
A simple and highly efficient strategy has been developed for the synthesis of 2-amidobenzoic acids through the tert-butyl hydroperoxide (TBHP)-mediated oxygenation and sequential ring opening of 2-arylindoles in a one-pot fashion under metal-free aerobic conditions. The developed synthetic protocol is operationally simple, tolerates a wide range of functional groups, and is amenable to the gram-scale. Radical trapping experiments revealed that the reaction involves a radical pathway. The synthesized compounds (2a–s) were tested for in vitro antimicrobial activity. Among all screened compounds, 2d showed the maximum antibacterial activity against P. aerugunosa (ZOI = 17 mm, MIC = 32 μg mL−1) and compounds 2d and 2p showed the maximum (32 μg mL−1) antifungal activity against A. flavus and C. albicans.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...