Synthesis of β-sulfinyl cyclobutane carboxylic amides via a formal α to β sulphoxide migration process†
Abstract
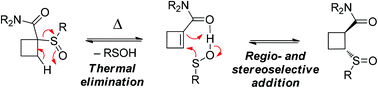
An original tandem reaction consisting of a thermal elimination–addition process was developed. Highly substituted β-sulfinyl cyclobutane carboxylic acid derivatives were obtained from isomeric α-sulfinyl derivatives in a single operation in good to high yields and with high trans diastereoselectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...