Highly functionalised (γ-azido/γ-fluoro-β-iodo/)vinyl derivatives from phosphorus based allenes or allenoates: I⋯O halogen bonding interactions†
Abstract
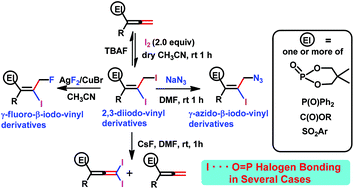
Multifunctional γ-azido/γ-fluoro-β-iodo-vinyl phosphine oxides/phosphonates/esters/sulfone were synthesised by iodination followed by azidation/fluorination of phosphorus-based allenes or allenoates (allenyl esters) or a sulphur based allene. Surprisingly, the reaction of (γ,β)-diiodo-vinyl-phosphonate with TBAF [n-Bu4NF] led to the corresponding allenylphosphonate; in contrast, the use of CsF in a similar reaction led to novel γ-diiodo-allenylphosphonate along with the corresponding non-halogenated allenylphosphonate. The combination AgF2/CuBr could be used to obtain the γ-fluoro-β-iodo-vinyl phosphine oxides and related phosphorus-free γ-fluoro-β-iodo-vinyl esters. In many cases, I⋯O halogen to oxygen non-covalent bonding interactions (‘halogen bonding’) involving the phosphoryl (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) oxygen, as evidenced by single crystal X-ray crystallography, are also observed.
O) oxygen, as evidenced by single crystal X-ray crystallography, are also observed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...