One-pot ortho-amination of aryl C–H bonds using consecutive iron and copper catalysis†
Abstract
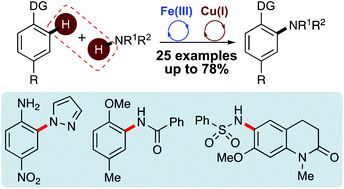
A one-pot approach for ortho-coupling of arenes with non-actived N-nucleophiles has been developed using sequential iron and copper catalysis. Regioselective ortho-activation of anisoles, anilines and phenols was achieved through iron(III) triflimide catalysed iodination, followed by a copper(I)-catalysed, ligand-assisted coupling reaction with N-heterocycle, amide and sulfonamide-based nucleophiles. The synthetic utility of this one-pot, two-step method for the direct amination of ortho-aryl C–H bonds was demonstrated with the late-stage functionalisation of 3,4-dihydroquinolin-2-ones. This allowed the preparation of a TRIM24 bromodomain inhibitor and a series of novel analogues.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...