Friedel–Crafts alkylation with α-bromoarylacetates for the preparation of enantioenriched 2,2-diarylethanols†
Abstract
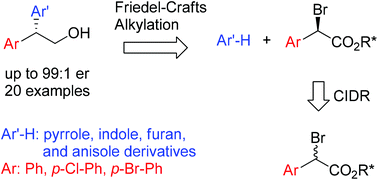
Highly enantioenriched 2,2-diarylethanols can be efficiently synthesized through the Friedel–Crafts alkylation of (hetero)arenes with configurationally labile α-bromoarylacetates. The substitution of highly diastereoenriched α-bromoarylacetates occurs in the presence of AgOTf, and the subsequent reduction affords diverse 2,2-diarylethanols with high yields and enantioselectivities up to 99 : 1 er. In addition, the application of this asymmetric synthetic methodology to the preparation of highly enantioenriched dihydrobenzofuran and indoline derivatives is demonstrated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...