Perylenequinonoid-catalyzed photoredox activation for the direct arylation of (het)arenes with sunlight†
Abstract
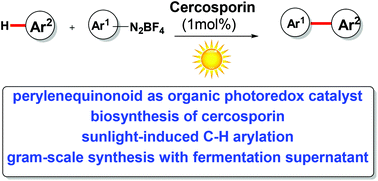
Naturally occurring perylenequinonoid pigments (PQPs) have attracted considerable attention owing to their excellent properties of photosensitization. They have been widely investigated as an aspect of photophysics and photobiology. However, their applications in photocatalysis are yet to be explored. We report here that sunlight along with 1 mol% cercosporin, which is one of the perylenequinonoid pigments, catalyzes the direct C–H bond arylation of (het)arenes by a photoredox process with good regioselectivity and broad functional group compatibility. Furthermore, a gram-scale reaction with great conversions of substrates was achieved even by a cercosporin-containing supernatant without organic solvent extraction and purification after liquid fermentation. Thus we set up a bridge between microbial fermentation and organic photocatalysis for chemical reactions in a sustainable, environmentally friendly manner.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...