Solvent-dependent photophysics of a red-shifted, biocompatible coumarin photocage†
Abstract
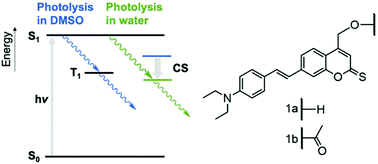
Controlling the activity of biomolecules with light-triggered photocages is an important research tool in the life sciences. We describe here a coumarin photocage that unusually combines the biocompatible optical properties of strong absorption at a long wavelength close to 500 nm and high photolysis quantum yields. The favourable properties are achieved by synthetically installing on the photocage scaffold a diethyl amino styryl moiety and a thionoester group rather than the lactone typical for coumarins. The photocage's photophysics are analysed with microsecond transient absorption spectroscopy to reveal the nature of the excited state in the photolysis pathway. The excited state is found to be strongly dependent on solvent polarity with a triplet state formed in DMSO and a charge-separated state in water that is likely due to aggregation. A long triplet lifetime is also correlated with a high photolysis quantum yield. Our study on the biocompatible photocage reveals fundamental insight for designing advanced photocages such as longer wavelengths in different solvent conditions tailored for applications in basic and applied research.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...