Synthesis of solandelactone F, constanolactone A and an advanced intermediate towards solandelactone E from a common synthetic intermediate†
Abstract
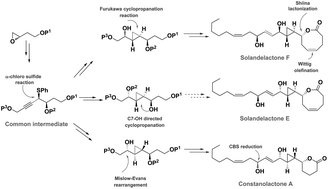
The stereoselective synthesis of solandelactone F, constanolactone A and an advanced intermediate towards solandelactone E, from a common synthetic intermediate, is disclosed. The propargylic sulfide stereocenter is created stereoselectively via carbon–carbon bond formation in the reaction of α-chloro sulfides with alkynylzinc reagents via 1,2-asymmetric induction by a β-siloxy group. The characteristic 1,4-diol motif of the natural products is introduced by a [2,3] sigmatropic rearrangement of an allylic sulfoxide or by the Mislow–Evans–Braverman rearrangement of a propargylic sulfoxide followed by stereoselective reduction of the ensuing α,β-unsaturated ketone. Unlike earlier reports, the C11/C9 carbinol center is created with excellent stereocontrol and derivatives of natural products differing at C14/C12 can be readily obtained. Catalytic asymmetric protocols and substrate-controlled asymmetric induction are utilized for the efficient introduction of the stereogenic centers.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...