Aryl–aryl coupling in a polycyclic aromatic hydrocarbon with embedded tetracoordinate boron centre†
Abstract
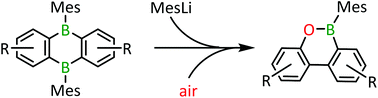
The addition of 2 eq. of MesLi to the biphenylene-containing 9,10-dihydro-9,10-diboraanthracene (DBA) 2 results in the formation of the corresponding oxaboraphenanthrene 3 after column chromatography under ambient conditions. In the initial step, the anionic B–Mes monoadduct of 2 is generated, which eliminates a formal [Mes2B:]− ion with concomitant C–C-bond formation (room temperature, 18 h). The resulting biphenylene-containing borafluorene 4 is still sufficiently Lewis acidic to add the second equivalent of MesLi ([4Mes]−). Using pristine 9-mesityl-9-borafluorene as a model system, we confirmed that both 4 and [4Mes]− should be capable of inserting an oxygen atom to furnish the observed oxaboraphenanthrene scaffold. The biphenylene-containing oxaboraphenanthrene 3 is a yellow compound with an absorption maximum at λmax = 450 nm. Similar to its DBA analogue 2, 3 shows no photoluminescence.
- This article is part of the themed collection: Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...