Catalyst-free and selective trifluoromethylative cyclization of acryloanilides using PhICF3Cl†
Abstract
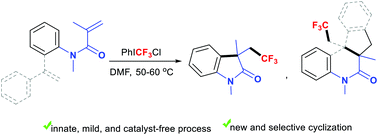
Trifluoromethylation-triggered cyclization of alkenes provides a useful route to CF3-containing cyclic compounds. Current approaches to generate CF3-based initiators from a CF3 source require a catalyst or an activator. This work describes a catalyst-free protocol to innately produce electrophilic CF3 species from PhICF3Cl for trifluoromethylative cyclization of acryloanilides. A new domino biscyclization of dienes has been developed leading to trifluoroethylated tetrahydroindenoquinolinones with chemo- and stereo-selectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...