A chiral bicyclic skeleton-tethered bipyridine–Zn(OTf)2 complex as a Lewis acid: enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes†
Abstract
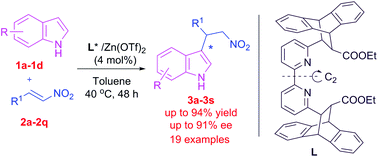
A conformationally rigid chiral bicyclic skeleton tethered bipyridine–Zn(OTf)2 complex facilitated the enantioselective Friedel–Crafts alkylation of indoles with trans-β-nitroarylalkenes in an enantioselective manner at elevated temperature. Indoles reacted smoothly with β-nitroarylalkenes to generate the corresponding 3-(2-nitroalkyl)indoles in good to excellent yields (up to 94%) with moderate to excellent enantioselectivities (up to 91%). The stereochemical outcome of the product from indole and trans-β-nitrostyrene in the presence of the CRCB tethered bipyridine–Zn(OTf)2 complex and the DFT calculation of the CRCB tethered bipyridine–Zn:trans-β-nitrostyrene complex support the si-face attack of indole on trans-β-nitrostyrene.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...