Lacto-N-tetraose synthesis by wild-type and glycosynthase variants of the β-N-hexosaminidase from Bifidobacterium bifidum†
Abstract
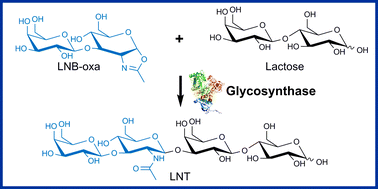
Lacto-N-biose 1,2-oxazoline was prepared chemo-enzymatically and shown to be a donor substrate for β-1,3-glycosylation of lactose by the wild-type and glycosynthase variants (D320E, D320A, Y419F) of Bifidobacterium bifidum β-N-hexosaminidase. Lacto-N-tetraose, a core structure of human milk oligosaccharides, was formed in 20–60% yield of donor substrate (up to 8 mM product titre), depending on the degree of selectivity control by the enzyme used.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...