Reaction condition controlled nickel(ii)-catalyzed C–C cross-coupling of alcohols†
Abstract
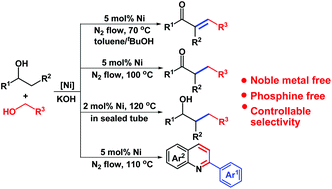
The challenge in the C–C cross-coupling of secondary and primary alcohols using acceptorless dehydrogenation coupling (ADC) is the difficulty in accurately controlling product selectivities. Herein, we report a controlled approach to a diverse range of β-alkylated secondary alcohols, α-alkylated ketones and α,β-unsaturated ketones using the ADC methodology employing a Ni(II) 4,6-dimethylpyrimidine-2-thiolate cluster catalyst under different reaction conditions. This catalyst could tolerate a wide range of substrates and exhibited a high activity for the annulation reaction of secondary alcohols with 2-aminobenzyl alcohols to yield quinolines. This work is an example of precise chemoselectivity control by careful choice of reaction conditions.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...