Thiosquaramide-catalysed asymmetric double Michael addition of 2-(3H)-furanones to nitroolefines†
Abstract
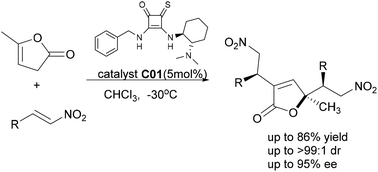
New chiral thiosquaramides and their applications in catalytic asymmetric double addition of 5-methyl-2(3H)-furanones to nitroolefins were described. Enantiomerically enriched 2,4,4-trisubstituted butenolides bearing a quaternary stereogenic center could be smoothly constructed with high diastereoselectivities (up to >99 : 1 dr) and excellent enantioselectivities (up to 95% ee) under mild conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...