Bifunctional organocatalysts for the conversion of CO2, epoxides and aryl amines to 3-aryl-2-oxazolidinones†
Abstract
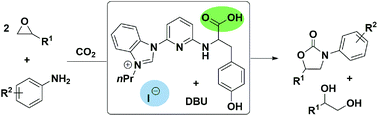
A route to synthesize 3-aryl-2-oxazolidinones is developed, which is achieved through a three component reaction between CO2, aryl amines, and epoxides with a binary organocatalytic system composed of organocatalysts and DBU (1,8-diazabicyclo[5.4.0]undec-7-ene). The method allows wide scopes of epoxide and aryl amine substrates with various functional groups under mild reaction conditions. The control experiments indicate that a cyclic carbonate is formed via cycloaddition of epoxides with CO2, which further reacts with the β-amino alcohol originating from epoxides and aryl amines, resulting in the formation of 3-aryl-2-oxazolidinones finally.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...