Solid phase syntheses of peptoid like arylureido compounds and sequencing of isobars without molecular encoding†
Abstract
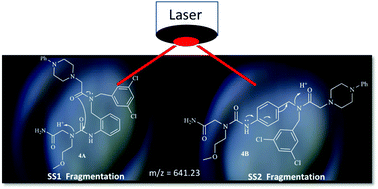
Arylureido-backbone containing peptoid-like trimers were prepared using the one-bead-one-compound approach. Isobaric molecules were synthesized from isocyanate precursors that contain alkyl halide handles at the ortho and para-positions in the phenyl ring. After chain extension with a primary amine, the piperazine-capped molecules were sequenced using tandem mass spectrometry and successfully identified based on their fragmentation pattern without a need for internal molecular encoding.


 Please wait while we load your content...
Please wait while we load your content...