Lewis base catalyzed regioselective cyclization of allene ketones or α-methyl allene ketones with unsaturated pyrazolones†‡
Abstract
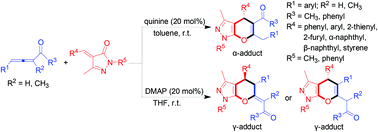
A nitrogen-containing Lewis base catalyzed highly regioselective [4 + 2] cycloaddition of allene ketones or α-methyl allene ketones with unsaturated pyrazolones has been disclosed to give the corresponding tetrahydropyrano [2,3-c] pyrazoles in moderate to good yields under mild conditions. High regioselectivity, 100% atom-economy, broad substrate scope and good functional group tolerance are attractive features of this process and make it a practical and versatile transformation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...