Zinc-catalyzed regioselective C–P coupling of p-quinol ethers with secondary phosphine oxides to afford 2-phosphinylphenols†
Abstract
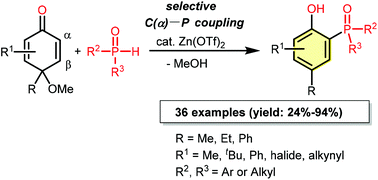
The zinc triflate-catalyzed highly regioselective C–P cross coupling reaction of p-quinol ethers with secondary phosphine oxides is reported. The reaction provides a facile alternative method for the synthesis of 2-phosphinylphenols in good to high yields. Mechanistically, zinc triflate may serve as an oxophilic σ-Lewis acid to activate the C–O bond in p-quinol ether first. Then the regioselective attack of the phosphorus nucleophile at the α-carbon position takes place to form the C–P bond and give the product. In addition, α-alkynyl substituted p-quinol ethers also react with secondary phosphine oxides in the same reaction mode to give 6-alkynyl 2-phosphinylphenols in the presence of the zinc catalyst.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...