The systematic influence of solvent on the conformational features of furanosides†
Abstract
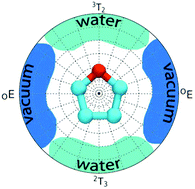
The endo- and exo-anomeric effects are the two most recognizable stereoelectronic effects exhibited by carbohydrates. Their presence relies on the interactions between ring substituent(s) and ring oxygen atoms. Here, we report the finding of a new effect that partially controls the conformational properties of furanose rings and can be ascribed to the influence of the solvent on the electronic structure of a molecule. In contrast to anomeric effects, it is not dependent on either presence or absence of ring substituents. Its origins lie in a solvent-induced flux of atomic charges that involves atoms forming the furanose ring. This systematically changes the energy of the whole molecular system and alters the ring-distortion free energies by ∼2.5–6.5 kJ mol−1, favoring the geometries close to the twist 3T2/2T3 conformers and disfavoring the envelope OE/EO-like shapes. This intriguing effect has never been reported before, although it is expected to exist in all furanose rings. Along with more recognized stereoelectronic effects, this phenomenon contributes to a wide applicability of the two-state (north vs. south) model of pseudorotation in furanosides and, in the case of extremely flexible furanose rings, may change the preferred conformation type in comparison with the gas-phase-oriented predictions.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...