DFT mechanistic investigation into phenol dearomatization mediated by an iodine(iii) reagent†
Abstract
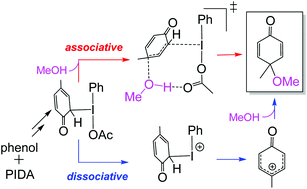
Density functional theory (DFT) was utilized to investigate the mechanistic aspects of the oxidative dearomatization of phenols mediated by an iodine(III) reagent. In this article, we will show that the conventional mechanism in which an iodine(III) phenolate is proposed as the key intermediate is not operative, and the process is promoted if the phenolate ligand is dearomatized on the iodine(III) center. The dearomatized phenolate is calculated to be a more potent reductant than phenolate itself. In such a case, the reaction is capable of proceeding via two competitive mechanisms (dissociative and associative). Consistent with the experimental findings, we found that while the less polar solvents considerably disfavor the dissociative mechanism, they have an insignificant effect on the associative one. The energetic order of these two mechanisms is calculated to be influenced by the nature of the counter anion coordinated to the iodine(III) center.


 Please wait while we load your content...
Please wait while we load your content...