Synthesis of α-arylthioacetones using TEMPO as the C3 synthon via a reaction cascade of sequential oxidation, skeletal rearrangement and C–S bond formation†
Abstract
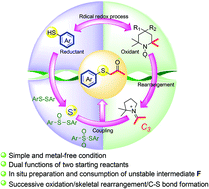
Here, we present an unprecedented pathway to α-sulfenylated carbonyl compounds from commercially available thiols and universally employed TEMPO and its analogues, which act as C3 synthons through skeletal rearrangement under simple and metal-free conditions. Mechanism studies suggest that this reaction involves a consecutive radical oxidation and cation coupling process. TEMPO analogues and thiols serve as oxidants and reductive reagents, respectively, along the radical process, while in the coupling process, the former ones afford C3 synthons to couple with related sulfur sources.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...