Mechanism and chemoselectivity origins of bioconjugation of cysteine with Au(iii)-aryl reagents†
Abstract
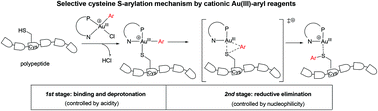
Organometallic reagents, in particular Pd(II)- and Au(III)-aryl reagents, have recently emerged as an efficient tool for bioconjugation. However, the detailed mechanism and origins of chemoselectivity are not well established, but are highly desirable from both synthetic and theoretical viewpoints. In this paper, we report that a computational study dealing with the reaction mechanism of Au(III)-aryl reagents enabled selective cysteine S-arylation of peptides and proteins developed by Maynard and Spokoyny et al. (J. Am. Chem. Soc., 2018, 140, 7065). Our calculation results suggest that the reaction proceeds by a cationic Au(III)/Au(I) pathway involving elementary steps of (a) binding of the SH residue to the Au(III) center, (b) deprotonation of the SH residue, and (c) reductive elimination from a key four-coordinate square planar (L)Au(III)(thiolate)(Ar) (L is a P,N-bidentate ligand) intermediate. Furthermore, the chemoselectivity of S-arylation against arylation of other nucleophilic residues can be rationalized in terms of energy demand of the three elementary steps. For instance, amine N-arylation is more difficult than S-arylation due majorly to the much higher energy required for deprotonation of much more basic N–H bonds than for deprotonation of weakly acidic S–H bonds. Carboxylate O-arylation is challenging due to the high activation energy of reductive elimination from LAu(III)(carboxylate)(aryl), because carboxylate is much less nucleophilic than thiolate. These results thus identify acidity and nucleophilicity of the residue as two inherent factors for bioconjugation. This study provides a useful and convenient approach for predicting and rationalizing the feasibility and chemoselectivity of related bioconjugation reactions.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...