Synthesis of diphenylamine macrocycles and their anti-inflammatory effects†
Abstract
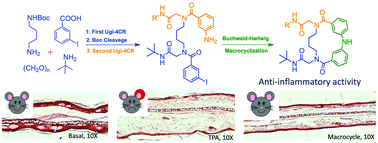
A collection of fourteen diphenylamine macrocyclic derivatives containing a peptide chain with different substituents was synthesized using a protocol of two Ugi four component reactions (Ugi-4CR) and a Buchwald–Hartwig macrocyclization. Their anti-inflammatory effects were assayed with an ear edema model using 12-O-tetradecanoylphorbol-13-acetate, while the activity of myeloperoxidase was determined to evaluate the index of leukocyte infiltration. Compound 5e had an ID50 of 0.18 μM per ear with a potency higher than that of the reference drugs indomethacin and celecoxib (0.24 and 0.91 μM per ear, respectively). Moreover, the cytotoxicity of the macrocycles was determined in two healthy cell lines, showing a low percentage of toxicity.
- This article is part of the themed collections: Celebrating Latin American Talent in Chemistry and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...