A metal- and base-free domino protocol for the synthesis of 1,3-benzoselenazines, 1,3-benzothiazines and related scaffolds†
Abstract
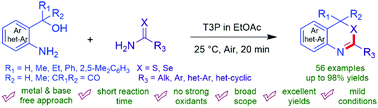
Efficient protocols have been described for the synthesis of 1,3-benzoselenazines, 1,3-benzothiazines, 2-aryl thiazin-4-ones and diaryl[b,f][1,5]diazocine-6,12(5H,11H)-diones. These transformations were successfully driven towards the product formation under mild acid catalyzed reaction conditions at room temperature using 2-amino aryl/hetero-aryl alkyl alcohols and amides as substrates. The merits of the present methods also rely on the easy access of rarely explored bioactive scaffolds like 1,3-benzoselenazine derivatives, for which well-documented methods are rarely known in the literature. A broad range of substrates with both electron-rich and electron-deficient groups were well-tolerated under the developed conditions to furnish the desired products in yields up to 98%. The scope of the devised method is not only restricted to the synthesis of 1,3-benzoselenazines, but it was also further extended towards the synthesis of 1,3-benzothiazines, 1,3-benzothiazinones and the corresponding eight membered N-heterocycles such as diaryl[b,f][1,5]diazocine-6,12(5H,11H)-diones.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...