Efficient metal-free aminoiodination of alkenes with N-fluorobenzenesulfonimide under mild conditions†
Abstract
A novel efficient metal-free aminoiodination of alkenes with N-fluorobenzenesulfonimide (NFSI) through an iodonium intermediate under mild conditions with good regioselectivity and stereoselectivity is reported. Unlike transition-metal catalysed aminative bisfunctionalization with NFSI in which the oxidative addition of NFSI to transition-metals affords an electrophilic amino radical, the oxidation of anionic iodide by NFSI in situ generates an electrophilic iodine cation and an amino nucleophile to fulfil this efficient reaction. 2,2,6,6-Tetramethyl-piperidine-1-oxyl (TEMPO) could considerably promote this iodoamination at room temperature. A preliminary trial suggests that bromoamination could also be achieved under similar conditions.
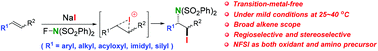
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...