tert-Butyl nitrite promoted transamidation of secondary amides under metal and catalyst free conditions†
Abstract
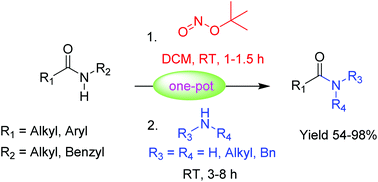
A mild and efficient method is demonstrated for the transamidation of secondary amides with various amines including primary, secondary, cyclic and acyclic amines in the presence of tert-butyl nitrite. The reaction proceeds through the N-nitrosamide intermediate and provides the transamidation products in good to excellent yields at room temperature. Moreover, the developed methodology does not require any catalyst or additives.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...