TBAI-catalyzed selective synthesis of sulfonamides and β-aryl sulfonyl enamines: coupling of arenesulfonyl chlorides and sodium sulfinates with tert-amines†
Abstract
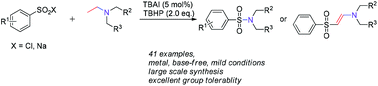
A simple, practical and metal-free method has been developed for the synthesis of sulfonamides and β-arylsulfonyl enamines via the selective cleavage of C–N and C–H bonds through the iodine-catalyzed oxidation of arenesulfonyl chlorides and sodium sulfinates with tert-amines. The method uses commercially available inexpensive catalysts and oxidants, and has a wide substrate scope and operational simplicity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...