Highly selective AlCl3 initiated intramolecular α-alkylation of α,β-unsaturated lactams and lactones†
Abstract
An unprecedented example of AlCl3 initiated intramolecular α-alkylation of α,β-unsaturated lactams and lactones is reported. A variety of substrates containing an intramolecular diene yield exclusively regioselective six-membered ring products. This reaction protocol generates a new stereo centre which may be of high interest for the functionalization of bioactive coumarin and quinolinone derivatives.
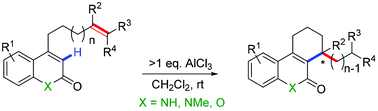


 Please wait while we load your content...
Please wait while we load your content...