Applications of chiral naphthyloxycyclohexanols in deracemization of α-substituted carboxylic acids by dynamic thermodynamic resolution†
Abstract
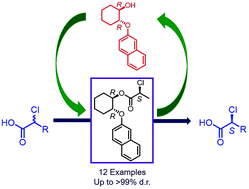
Two derivatives of trans-2-naphthyloxycyclohexanol were synthesized, their enantiomers were separated by enzyme mediated kinetic resolution and their absolute configuration was established by synthesizing their diastereomers with esters of known chiral description. Chiral alcohols were then used as chiral auxiliaries for the preparation of esters by coupling with racemic α-halo acids. During the coupling reactions with DCC and a suitable base, an efficient dynamic thermodynamic resolution was observed and the products were isolated in high diastereomeric purity. The effect of several parameters on the reaction was studied and the absolute configuration of a newly created chiral centre was established by single crystal X-ray analysis; the correlation of the structure of chiral auxiliary and diastereoselectivity was investigated. The observed diastereoselectivity was in accordance with the relative energy profile of the products. The chirally pure α-halo acid could be separated from the auxiliary, without any loss of optical purity of both components.


 Please wait while we load your content...
Please wait while we load your content...