I2/TBHP-Mediated tandem cyclization and oxidation reaction: Facile access to 2-substituted thiazoles and benzothiazoles†
Abstract
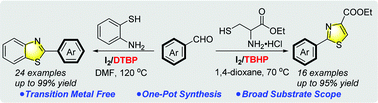
The efficient synthesis of 2-substituted thiazoles and benzothiazoles has been accomplished employing readily available cysteine esters and 2-aminobenzenethiols as N and S sources. The reaction proceeds under an I2/TBHP system and involves a one-pot tandem cyclization and oxidation sequence. A diverse range of aldehydes is amenable for this transition-metal-free protocol, which provides an alternative method to rapidly access thiazole-containing molecules.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...