Synthesis of A-ring quinolones, nine-membered oxolactams and spiroindoles by oxidative transformations of 2,3-indolotriterpenoids†
Abstract
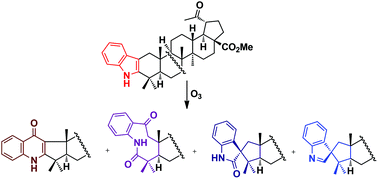
This paper describes an access to new nitrogen-containing heterocyclic triterpenoids by the reaction of 2,3-indolotriterpenoids with ozone and dimethyldioxirane. The oxidation of indolo-fused 28-oxo-allobetulin or methyl platanoate with ozone led to a mixture of a quinolone as the major product and a nine-membered 2,3-seco-2-oxolactam and three different types of spiroindoles as byproducts. The formation of quinolone and 2,3-seco-2-oxolactam derivatives could be explained by the standard 1,3-dipolar cycloaddition of ozone to the C2(3)-double bond of the triterpene core similar to the products observed in the ozonolysis of indoles in the Witkop–Winterfeldt oxidation (WWO). The formation of spiroindoles was unexpected and could be explained through the 1,2-cycloaddition of ozone to the C2(3)-double bond with consecutive intramolecular rearrangements of the 2,3-epoxy-intermediate. These spiroindoles seem to be novel structures observed in the WWO reaction. The formation of only two isomeric triterpene spiroindolinones was achieved by the oxidation of 2,3-indolo-28-oxo-allobetulin with dimethyldioxirane that could be explained by the rearrangement of the 2,3-epoxy-intermediate. 19β,28-Epoxy-18α-olean-28-oxo-2-nor-2,3-4′(1H)-quinolone was the most active against HPV-11 with EC50 0.45 μM and SI50 322 in a primary assay and SI90 < 10 against HPV-16 in a secondary assay. The oxidative transformations of indolotriterpenoids have great potential for further modifications towards the preparation of new biologically active compounds.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...