Gold-catalysed regioselective cascade cycloisomerisation reactions of aza-enediynes for the synthesis of substituted benzoisoquinoline derivatives†
Abstract
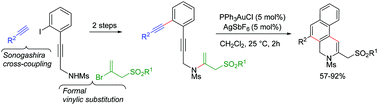
Aza-enediynes underwent a facile, regioselective gold-catalysed cascade cycloisomerisation to furnish dihydrobenzo[f]isoquinoline derivatives in excellent yields. The aza-enediynes were conveniently prepared via a formal vinylic displacement reaction of allyl bromosulfones. The latter functioned as a stable and easily accessible synthetic equivalent of allenyl sulfone.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...