Tandem synthesis of 4-aminoxanthones is controlled by a water-assisted tautomerization: a general straightforward reaction†
Abstract
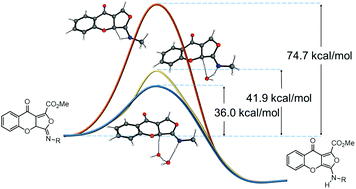
Aminoxanthones constitute a group of therapeutically promising compounds that so far have been synthetically challenging. Here, we report the synthesis of both aminodihydroxanthones and fully aromatized aminoxanthones by an easy to perform, one-step multicomponent reaction of isocyanides, 3-carbonylchromones and dienophiles. The mechanism of the reaction involves a sequence of a [4 + 1] cycloaddition, iminolactone-aminofuran tautomerization, [4 + 2] cycloaddition, oxygen ring opening and aromatization. Remarkably, DFT quantum chemical computations revealed that the iminolactone-aminofuran tautomerization requires the assistance of a water molecule and, contrary to intuition, it is the rate-determining step. Conversely, both the [4 + 1] and the [4 + 2] cycloadditions have relatively low calculated energy barriers, regardless the substituents on the starting materials. Thus, we have stablished a straightforward and a wide-ranging synthesis of diversely substituted xanthones. This highly convergent process has also been applied to the synthesis of biologically important chromenophenantridines and secalonic acid related xanthone dimers.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...