Site-selectivity control in hetero-Diels–Alder reactions of methylidene derivatives of lawsone through modification of the reactive carbonyl group: an experimental and theoretical study†
Abstract
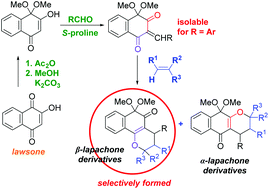
A new perspective on the reactivity of hydroxyquinones was revealed as an acetal derivative of lawsone was synthesized, isolated, and used in tandem Knoevenagel/hetero-Diels–Alder reactions catalyzed by S-proline. The intermediate alkylidene-1,3-diones that were formed in situ reacted with electron rich alkenes to predominantly afford pyrano-1,2-naphthoquinone (β-lapachone) derivatives along with the isomeric pyrano-1,4-naphthoquinone (α-lapachone) derivatives in high to excellent total yields. Interestingly, the highly reactive arylidene-1,3-dione derivatives were found to be stable and isolable. DFT calculations suggest that these hetero-Diels–Alder reactions have a high polar character, taking place through a two-stage one-step mechanism. An analysis of the conceptual DFT indices allows explaining the remarkable site-selectivity observed.


 Please wait while we load your content...
Please wait while we load your content...