Iron-catalyzed protodehalogenation of alkyl and aryl halides using hydrosilanes†
Abstract
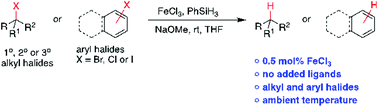
A simple and efficient iron-catalyzed protodehalogenation of alkyl and aryl halides using phenylhydrosilane is disclosed. The reaction utilizes FeCl3 without the requirement of ligands. Unactivated alkyl and aryl halides were successfully reduced in good yields; sterically hindered tertiary halides were also reduced including the less reactive chlorides. The scalability of this methodology was demonstrated by a gram-scale synthesis with a catalyst loading as low as 0.5 mol%. Notably, disproportionation of phenylsilane leads to diphenylsilane that further reduces the halides. Preliminary mechanistic studies revealed a non-radical pathway and the source of hydrogen is PhSiH3via deuterium labeling studies. Our methodology represents simplicity and provides a good alternative to typical tin, aluminum and boron hydride reagents.
- This article is part of the themed collections: Catalysis & biocatalysis in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...