Stereo- and regioselective gold(i)-catalyzed hydroamination of 2-(arylethynyl)pyridines with anilines†
Abstract
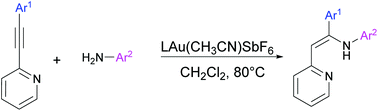
The gold-catalyzed hydroamination of 2-(arylethynyl)pyridines with anilines affords stereoselectively Z-enamine products with excellent regioselectivity. The reaction proceeds with moderate to excellent yields and accommodates a diverse range of functional groups on alkynes (ether, bromo, trifluoromethyl, acetyl, and carbomethoxy) and anilines (ether, bromo, chloro, and carbethoxy). The stereochemistry of the obtained enamines is complementary to that reported in previous studies. A plausible explanation for the observed selectivity was attained by means of NMR experiments.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...