Chemoselective reduction of isothiocyanates to thioformamides mediated by the Schwartz reagent†
Abstract
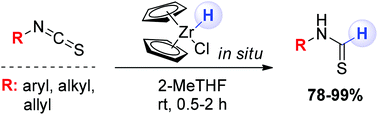
Thioformamides are easily prepared – under full chemocontrol – through the partial reduction of isothiocyanates with the in situ generated Schwartz reagent. The high electrophilicity of the starting materials enables the straightforward addition of the hydride ion, thus constituting a reliable and high-yielding method for obtaining variously functionalized thioformamides. Sensitive chemical groups to the reduction conditions such as nitro, ester, alkene, azo, azide and keto groups do not interfere with the chemoselectivity of the process. Moreover, the stereochemical information embodied in the starting material is fully retained in the final products. The synthetic potential of the selected thioformamide template is also briefly discussed.
- This article is part of the themed collections: Synthetic methodology in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...